The Sixera Pharma Project
The Project
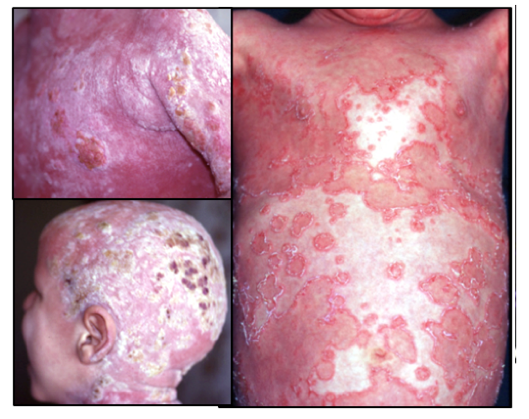
Netherton syndrome
It's a rare reason
Netherton syndrome (NS) is a rare genetic disease leading to severe skin inflammation and allergic manifestations. The number of newborns affected by the disease is estimated to be 5 per million worldwide, although some patients are never properly diagnosed due to the rarity of the disease.
NS is characterized by a combination of three clinical manifestations:
- scaly redness of the skin (congenital ichtyosiform erythroderma)
- hair shaft abnormality; bamboo hair (tricorrhexis invaginata)
- constant atopic manifestations
Do you want to help us on our mission
If you are in a position to help our project or know someone that should be on our team you can contact us here.
Project breakdown
Step 1: accomplished
Step 1: accomplished
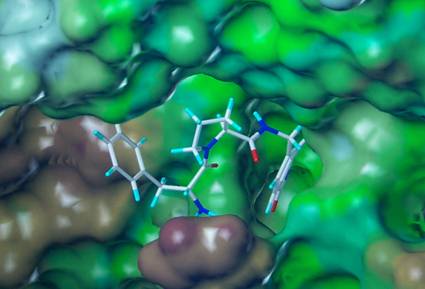
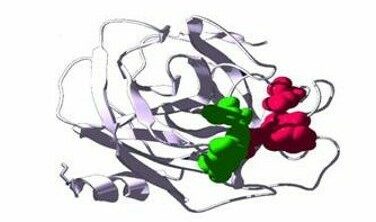
Image of the molecule
Develop small molecule inhibitors of KLK5, 7 and 14
- Through an extensive medicinal chemistry effort based on structural knowledge of KLK7, a number of compounds were designed and tested as specific inhibitors of the skin proteases KLK5, 7 and 14 that are causing the disease manifestaions.
- During screening of a large number of compounds, the most selective and potent inhibitors of KLK5, 7 and 14 were selected for further in vitro testing .
Step 2: accomplished
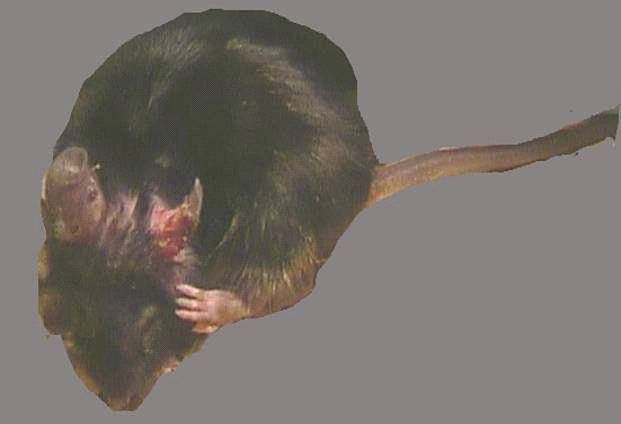
Picture of a mouse with a “netherton syndrome” like condition
Step 2: accomplished
Validate KLK5, 7 and 14 as targets in Netherton syndrome models
- In vitro testing of several different selective KLK5, 7 and 14 inhibitors was carried out.
- In situ zymography testing of compounds in Netherton syndrome skin sections was carried out to verify specificity.
- Further in vivo testing in a proprietary TG KLK7 mouse strain validated the effect of treatment with the selective protease inhibitors in this disease model.
Step 3: accomplished

here is how our cream is mixed in the factory
Step 3: accomplished
Toxicology testing and process scale up of lead compound SXR1096
- Toxicology testing of the lead compound was carried out to show that it is safe.
- A process for manufacture of the compound was developed and the compound produced at scale, also a formulation suitable for the sensitive skin of Netherton patients was developed and the compound in a cream formulation was produced at scale for use in a clinical trial .
Step 4: Ongoing
Clinical testing of a cream formulation of SXR1096 in a phase I/II study
- Together with leading clinics in Europe Sixera is now conducting a phase I/II clinical trial with our cream formulation of SXR1096 in Netherton patients .

